Social Survey Research Information Co., Ltd.
NEWS
Social Survey Research Information Co., Ltd.
Analysis of the potential for administering new drugs in terms of the number of patients
- from PatientsMap2022JP version -
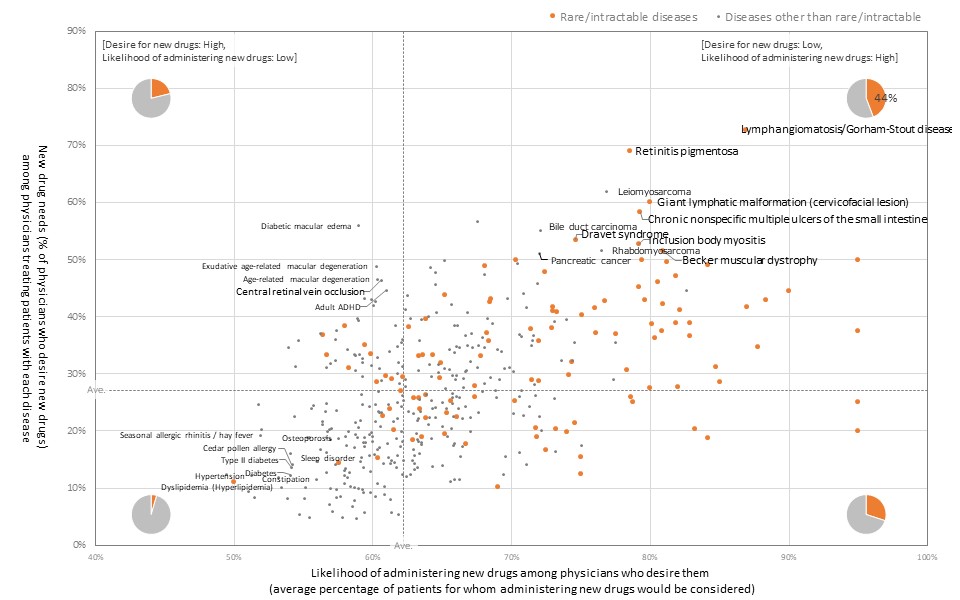
Forty-four percent of diseases for which there are strong needs for new drugs and high expected percentages of patients to be administered the new drugs are rare/intractable diseases.
Diseases for which there are strong needs for new drugs but the potential for administering new drugs is limited are clarified.
Social Survey Research Information Co., Ltd., which has developed and marketed PatientsMap, a database of patient numbers based on physician surveys, has published the analysis results of the differences in the likelihood of administering new drugs based on the disease characteristics using data from the Japanese version of PatientsMap 2022.
The results of the survey revealed that 44% of the diseases for which there are strong needs for new drugs and a high likelihood of administering new drugs are rare/intractable diseases. Meanwhile, there also tends to be strong needs for new drugs for ophthalmologic diseases, but the potential for administering new drugs tends to be low.
Analysis Background
When considering unmet medical needs from the perspective of new drug development, it is important to not only identify diseases for which there are strong needs for new drugs but also market size.
Market size should take into account the likelihood that a new drug will be administered in addition to the number of patients who have that disease. By analyzing the PatientsMap data, we would like to identify the differences in disease characteristics that may lead to the administration of new drugs.
[New drug needs and potential for administering new drugs.]
(from PatientsMap2022JP version)
Conclusion
The results of the above analysis indicate that even for diseases for which there are strong needs for new drugs, the size of the market in which new drugs may actually be administered varies depending on the characteristics of the disease. It is suggested that these differences may be due to the limitations of the existing treatments or other factors such as safety, administration methods, and economic burden, which will be important indicators in the strategic planning and resource allocation for new drug development.
PatientsMap will continue to contribute to the identification of unmet medical needs and the advancement of healthcare via research on a variety of diseases.
What is PatientsMap?
PatientsMap is a large database that surveys, with the cooperation of physicians, about 400 diseases in terms of physician coverage, number of patients, unmet medical needs (diseases for which new drugs are desired, reasons for wanting new drugs, and percentage of patients for whom administering new drugs would be considered), and the activities of real sales reps (in-person) and e-detailing activities of pharmaceutical companies. The same survey frame has been used continuously for more than 10 years since 2010.
Overview of PatientsMap 2022 Japan Version (2022JP)
A survey was conducted of m3 panel physicians.
| Target respondents: | : | M3-registered physicians |
|---|---|---|
| Method | : | Online survey |
| Fieldwork period | : | May 10 to September 5, 2022 |
| Sample size collected | : | 20,219 sample |
| Number of conditions surveyed | : | 437 diseases |
| Survey item: | : | Total number of patients (in the past month) Number of patients seen by condition (in the past month) Number of patients who were prescribed drugs by condition (in the past month) Need for new therapy Percentage of patients for whom administering new drugs would be considered Reasons why new drugs are desired by condition Manufacturers that have visited (in the past month) Manufacturers that you have had a remote meeting with one of their sales reps (in the past month) Manufacturer that have provided you with information via the Internet (in the past month) Reliable manufacturers |
Contact Information
| Social Survey Research Information Co., Ltd. | ||
|---|---|---|
| : | patientsmap@ssri.com | |
Social Survey Research Information Co., Ltd.
| Established | April 1982 |
| Capital | 27 million JPY |
| President & CEO | Takashi Makita |
| Number of employees | 137 |
| Location | Head Office NMF Shinjuku East Bldg. 2F, 3F 10-5 Tomihisacho, Shinjuku-ku, Tokyo 162-0067 JAPAN |
| Osaka office Daimei Bldg. 4F, 4-6-3 Hirano-cho, Chuo-ku, Osaka 541-0046 JAPAN | |
| Affiliate companies | PD Research Co., Ltd. MDB Co. SSRI China Co., Ltd. (overseas subsidiary) SSRI Asia Pacific Co., Ltd. (overseas subsidiary) HIMA Research (overseas affiliate) |
| URL | https://www.ssri.com/ |
